UNITS
It is paramount in calculations to keep the units straight. For example, one does not want to end up with a velocity of meters, but rather of meters per second (m s-1). This is a trivial example, but when dealing with non-dimensional numbers, such as Reynolds or Froude numbers, it is important that all units cancel. Moreover, there are constants, like viscosity, where the units are not intuitive, and complex calculations, such that the end result is, say velocity. One check on whether the calculation is done correctly (all the right numbers in the numerator, and all the right ones in the denominator) is to check the units. It is a simple operation that is of great value to keep you out of trouble.
A simple example of a unit operation is to convert feet to meters:
![]() We
deal with the units and numbers separately.
We
deal with the units and numbers separately.
First, the numbers: ![]()
Now, the units: ![]() So we divide ft by ft, which cancels ft, in by in,
which cancels in and
So we divide ft by ft, which cancels ft, in by in,
which cancels in and
cm by cm, which cancels cm. Nothing is left but m in the numerator
or the final units left is m.
Our final and complete answer to the problem is 0.3048 m.
Lets say, we mess up our conversion from ft to m.
We were in a rush to get it done and we start with the equation,
![]()
We calculate the equation numerically, resulting in,
No, problem, we know that a foot is much smaller than a meter. But we check ourselves, anyway using the units.
![]() …..ft
cancel but we are left with
…..ft
cancel but we are left with 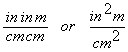
Whoops! We know something is wrong and we should go back and check.
Applications
1. What are the units of Boltzman's constant in the equation that relates heat flux, Q, to temperature, T?
Q = sT4 where Q
has units of watts per square meter (W m-2). We can look up in a table
that watts
are equivalent to (m2 kg s-3) and therefore Q has units of ( m2
kg s-3 m-2) or since the m cancel
the resulting units are (kg s-3).
T has units of degrees Kelvin (oK).
If we divide the units of Q by the units of T4, we will obtain the units of s.
s = Q T-4 is (kg s-3)(oK)-4, or (kg s-3 oK-4 another way to express this would be W m-2 oK-4 .
2. What are the units of shear stress?
t = r g h sin a ,
where r is density, g is gravity, and h is depth. a is a slope angle.
This equation expresses the shear stress, force per unit area in the slope direction as opposed to downward in the direction normal to the slope. This applies to water flow, avalanches, and to glacier flow, among other natural phenonmenon. Denser the medium, thicker the medium, or more steeply sloping the medium, greater the shear stress.
From what you have gained above, you should be able to show, in the m-kg-s metric system, that shear stress has units of kg s-2 m-1. Knowing that a Newton (N) is a unit of force (mass times acceleration) equivalent to N = kg m s-2 and area is m2, and shear stress is force per unit area, then we have N m-2.